Introduction
In spite of significant improvements in technology and hygienic practices at all stages of poultry production in developed countries, accompanied by advanced improvement in public sanitation, foodborne diseases remain a persistent threat to human and animal health. Foodborne diseases are still big issues of major concern in those countries. In developing countries, the need to produce sufficient food to meet the requirements of population increases, accompanied by bad economic situations often overshadow the need to ensure safe food products. Regardless of this fact, safe food is a fundamental requirement for all consumers, rich or poor. Food safety is not a discovery of recent times; it is a natural basic instinct of human survival. During human evolution, several approaches were adopted to achieve safety of food. One of the most famous approaches was practiced by several kings which would employ official and well trusted "tasters" that served as food safety sentinels for the kings and royal family members. Food safety and quality of food are currently big issues of major concern.
Many reports during recent years have shown that Salmonella and Campylobacter spp. are the most common causes of human foodborne bacterial diseases linked to poultry. In some areas also verotoxin producing Escherichia coli 0157:H7 (VTEC), Listeria and Yersinia have surfaced as additional foodborne pathogens causing human illness. Several other toxicogenic bacterial pathogens, such as Staphylococcus aureus, Clostridium perfringens, Clostridium botulinum and Bacillus cereus can also enter the human food chain via contaminated poultry carcasses. In addition, the development of antibiotic resistance in bacteria, which are common in both animals and humans, such as Methicillin Resistant Staphylococcus aureus (MRSA) and Extended-spectrum beta-lactamase (ESBL) bacteria, are also an emerging public health hazard.
Salmonella infection
Salmonella infections in poultry are distributed worldwide and result in severe economic losses when no effort is made to control them. In poultry, the genus Salmonella of the family Enterobacteriaceae, which include more than 2500 serovars, can roughly be classified into three categories or groups as follow: Salmonella can also be divided into three groups based on their host specificity and invasiveness [1]. Invasive salmonellas have the capability to “invade” the body from the intestinal lumen and thus infect organs, causing more serious disease. Group 1 contains serovars, which are highly host adapted and invasive. Examples are S. Gallinarum and S. Pullorum in poultry or S. Typhi in humans. Group 2 contains non-host adapted and invasive serovars. Salmonella in this group are of most concern regarding public health, since some of them are capable to infect humans and food producing animals and especially poultry can serve as reservoirs. There are approximately 10 – 20 serovars in this group. Currently, the most relevant serovars of them are S. Typhimurium, S. Enteritidis, S. Heidelberg, S. Hadar as well as S. Arizonae. Group 3 contains non-host adapted and non-invasive serovars, which are harmless for animals and humans. Most serovars of the genus salmonella belong to this group. Some serovars may be predominant for a number of years in a region or country. Then, they disappear and replaced by another serovars [2]. The infection can be transmitted vertically through contaminated eggs laid by infected carriers as well as horizontally spread (lateral). Hatcheries are one of the major sources of early horizontal transmission. Horizontal spread of Salmonella occurring during the hatching was shown in chickens, when contaminated and Salmonella-free eggs were incubated together. Salmonella can also spread through the hatchery by means of contamination of ventilation ducting, belt slots or door seals within hatchers, but may also result from infection and contamination that continuously recycles between hatchers, hatched birds, dust and crate washing equipment. During rearing the infection is transmitted horizontally (laterally) by direct contact between infected and uninfected flocks, and by indirect contact with contaminated environments through ingestion or inhalation of Salmonella organisms. Subsequently, there are many possibilities for lateral spread of the organisms through live and dead vectors. Transmission frequently occurs via faecal contamination of feed, water, equipment, environment and dust in which Salmonella can survive for long periods. Failure to clean and disinfect properly after an infected flock has left the site can result in infection of the next batch of birds. Significant reservoirs for Salmonella are man, farm animals, pigeons, waterfowl and wild birds. Rodents, pet’s insects and litter beetles (Alphitobius diaperinus) are also potential reservoirs and transmit the infection to birds and between houses [3]. Probably one of the most common sources for lateral spread of the organisms is feed. Nearly every ingredient ever used in the manufacture of poultry feedstuffs has been shown at one time or another to contain Salmonella. The organism occurs most frequently in protein from animal products such as meat and bone meal, blood meal, poultry offal, feather meal and fishmeal. Protein of vegetable origin has also been shown to be contaminated with Salmonella [4, 5].
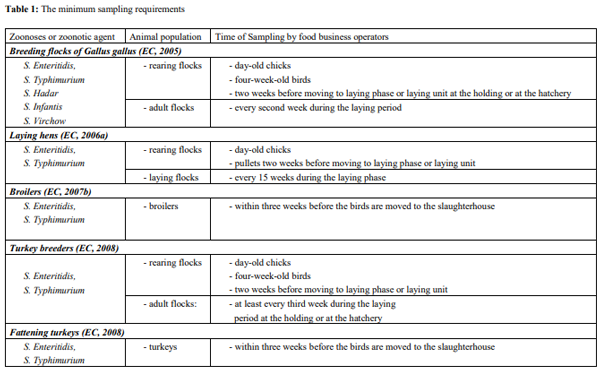
Since November 2003, several regulations from the European Parliament Council Regulation on the control of salmonella and other specified food-borne zoonotic agents were passed. This regulation covers the adoption of targets for the reduction of the prevalence of specified zoonosis in animal populations at the level of primary production, including breeding flocks (Chickens and turkeys), layers, broiler and turkey flocks. Food business operators must have samples taken and testing for the zoonosis and zoonotic agents especially Salmonella (Table 1) as summarized by Hafez (2010) [6].
Campylobacters
Thermophilic campylobacters are the most common bacterial cause of diarrhoea in humans worldwide. Enteric diseases caused by the thermophilic species C. jejuni, C. coli, C. lari, and C. upsaliensis range from asymptomatic infections to severe inflammatory bloody diarrhoea. The natural habitat of thermophilic Campylobacter is the intestinal tract of healthy birds and raw meat that can be contaminated during the slaughtering process [7]. It is estimated that as many as 90% of broilers and turkeys may harbour Campylobacter while showing little or no clinical signs of illness [8]. Poultry and poultry products remain the most common source of foodborne human campylobacteriosis. The major route for Campylobacter infection in poultry appears to be the horizontal transmission from the environment. Specific flocks that become infected show rapid rate of intra-house transmission and a high isolation rate from caecal swabs, water and litter. Campylobacter spp. are widespread in poultry not only during the growing period, but also on the poultry meat during slaughter and during processing of poultry products. Horizontal transmission is the most important mode of the introduction of Campylobacter into poultry flocks. However, the ability of Campylobacter to spread is limited by their relatively low tenacity, which can vary between strains. Especially dry environments kill Campylobacter within one or two hours [9].
Antibiotic resistant
The development of antibiotic resistance in bacteria, which are common in both animals and humans, is an emerging public health hazard. Controlling these foodborne organisms requires a broader understanding of how microbial pathogens enter and move through the food chain, as well as the conditions that promote or inhibit growth for each type of organism.
Multi-resistant bacteria are increasingly posing a hazard to human and animal health worldwide, impeding successful antibacterial treatment [10, 11]. In addition, the development of novel antibiotics does not keep step with the emergence of antimicrobial resistance in bacteria [12].
Among multi-resistant bacteria, vancomycin-resistant enterococci (VRE) have been estimated as one of the most common bacteria causing a rise in cases of nosocomial infections in humans in the last few years [10]. The prevalence of vancomycin-resistant enterococci (VRE) in 20 turkey flocks reared in the southwest of Germany was investigated. Enterococci were tested on the presence of the vancomycin resistance genes vanA, vanB (B1/B2/B3), and vanC (C1/C2/C3). Vancomycin-resistant enterococci were detected in 15 (75%) of the 20 turkey flocks investigated. In a total 68 isolates were isolated from birds and dust samples, enterococci bearing van-genes were detected. Of these, 12 isolates carried the vanA gene (17.6%) and 56 isolates carried the vanC1 gene (82.6%). Neither vanB (B1, B2, B3) genes nor the vanC2 or vanC3 genes could be detected [13].
In addition, Livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) have been isolated from a number of livestock species and persons involved in animal production. Turkey meat was also showed to be contaminated with MRSA [14]. Richter et al. investigated the prevalence of LA-MRSA in fattening turkeys and people living on farms that house fattening turkeys [15]. Eighteen (90%) of 20 investigated flocks were positive for MRSA. All-female flocks were positive, while 8 male flocks were positive. On 12 of the farms 22 (37.3%) of 59 persons sampled were positive for MRSA. None of them showed clinical symptoms indicative of an MRSA infection. People with frequent access to the stables were more likely to be positive for MRSA. In most flock’s MRSA clonal complex (CC) 398 were detected. In five flock’s MRSA of spa-type t002 were identified, which was not related to CC398. Moreover, other methicillin-resistant Staphylococcus spp. were detected on 11 farms and in 8 people working on the farms. Similar results were about MRSA in turkeys were published by El-Adway et al. [16].
Maasjost et al. investigated the antimicrobial susceptibility patterns of Enterococcus faecalis and Enterococcus faecium isolated from poultry flocks in Germany and they found that high resistance rates were identified in both Enterococcus species for lincomycin (72%–99%) and tetracycline (67%–82%) [17]. Half or more than half of Enterococcus isolates were resistant to gentamicin (54%–72%) and the macrolide antibiotics erythromycin (44%–61%) and tylosin-tartate (44%–56%). Enterococcus faecalis isolated from fattening turkeys showed the highest prevalence of antimicrobial resistance compared to other poultry production systems.
El- Adway et al. investigated 76 C. jejuni isolates were recovered from 67 epidemiologically unrelated meat turkey flocks in different regions of Germany in 2010 and 2011 [18]. Only one isolate was sensitive to all tested antibiotics. The numbers of isolates that were sensitive to streptomycin, erythromycin, neomycin, and amoxicillin were 69 (90.8%), 61 (80.2%), 58 (76.4%), and 44 (57.9%), respectively. The emergence of a high resistance rate and multidrug resistance to three or more classes of antimicrobial agents were observed. The resistance against sulphamethoxazole/trimethoprim, metronidazole, ciprofloxacin, naladixic acid, and tetracycline was 58 (76.3%), 58 (76.3%), 53 (69.7%), 51 (67.1%), and 42 (55.3%), respectively. Multidrug resistance to three or more classes of antimicrobial agents was found and ranged from 3.9% to 40.8%. Similar results were also found by examination of isolates collected from different free-range turkey flocks in Germany [19].
General approaches to control foodborne infections
To control the foodborne organisms, information is required to understand more fully, how microbial pathogens enter and move through the food chain, and the conditions, which promote or inhibit growth for each type of organism. In general, the main strategy to control foodborne infections in poultry should include monitoring, cleaning the production chain from the top, especially for vertically transmitted microorganism such as Salmonella by culling infected breeder flocks, hatching egg sanitation and limiting introduction and spread of infections at the farm level through effective hygiene measures [20-22]. An intensive and sustained rodent control is essential and needs to be well planned and routinely performed and its effectiveness should be monitored. In addition, reducing bacterial colonization by using feed additives such as short chain organic acids (formic acid, propionic acid), carbohydrates (lactose, mannose, galactose, saccharose), probiotics, competitive exclusion or use of vaccines are further possibilities [23, 24]. Live and inactivated vaccines are used to control Salmonella in poultry [25]. Generally, vaccination alone is of little value, unless it is accompanied by improvements in all aspects of management and biosecurity. In addition, further attention must be paid to the development of efficient vaccines against campylobacter infections.
Suggested linkSince the success of any disease control programme depends on the farm and personal sanitation, it is essential to incorporate education programmes about micro-organisms, modes of transmission as well as awareness of the reasons behind such control programmes by people involved in poultry production. In addition, effective education programmes must be implemented to increase public awareness of the necessary measures to be taken for protection against bacteria in food products from poultry.
Furthermore, in spite of significant improvement in technology and hygienic practices at all stages of food production accompanied with advanced improvement in public sanitation foodborne infections remains a persistent threat to human and animal health. The failure of the human population to apply hygienically acceptable food handling and cooking practice, and the fact that the processing plants are not able to reduce the level of pathogenic bacteria in poultry products, mean that every effort must be made to reduce the Salmonella contamination of the live birds before despatch to processing plants. New approaches to the problem of contamination must be adopted and the discussion on the decontamination of the end product must be re-evaluated carefully and without emotion. In addition, research must continue to find additional control and preventive means. Furthermore, the long term, development of poultry lines that are genetically resistant to some pathogens should be progressed.
Conclusions
Toward food safety in the EU several legislations are into force and their aims can be summarized according to Mulder (2011) as follows [26]:
- Safety (consumer health): by new methods to reduce the use of antibiotics/medicines; improve disease resistance; zoonosis control; traceability of animals and products
- Safety (product safety): stimulate and control hygienic processing, traceability of products and materials intended to come into contact with food
- Animal welfare: animals kept according to rules/systems
- Product quality: improved quality and composition; quality and chain control systems; traceability of animals and products.
- Environment: reducing environmental contamination, Nitrogen and Phosphorous. There is a critical look at the use of by-products of human food production. The re-use of by-products for non-food applications (feathers) should be encouraged.
- Rural impact, economic effects and biodiversity.