Introduction
With continuous increase in the economic value of the poultry industry, a deeper understanding of the nature and functioning of the avian immune system is important. The immune system enables the body to mount a defense against foreign organisms and antigens. However, the chicks’ immune system is underdeveloped and immature immediately after hatching, rendering them highly vulnerable to infectious threats present in the environment. The nutritionally balanced feeding program along with antibiotic growth promoters (AGPs’) in poultry diets played a significant role in achieving faster production success. Nevertheless, faster growth is likely to have a negative impact on the immune system due to decreased tolerance towards infectious diseases, thereby increasing the incidence of early chick mortality. In addition, the indiscriminate long-term usage of antibiotic growth promoters created a threat of bacterial resistance with major impacts on the future efficacy of these essential drugs. Consequently, due to the emergence of poultry welfare and environment protection concerns several countries have banned or reduced the prophylactic use of antibiotics as feed additives. This reduced the use of antibiotics in poultry production system, increased the demand for finding of alternate feed compounds along with a nutritious diet that can enhance gut health and immune function.
To address this need, a wide range of products are available in the market today which can help to modulate the innate immune system. One innovative approach that has been thoroughly studied is the use of beta-glucans in poultry diets. Although reports concerning the significance of beta-glucans in poultry are gaining popularity, still there seems to be some confusion in the poultry producers’ mind as to which source is better, how much is needed to improve immunity and what price is fair. The aim of this review was to give guidance on the major types of beta-glucans, their functional properties, and the option in choosing the best source with the potential to modulate the immune response in birds.
Sources and Structure of Beta-glucans
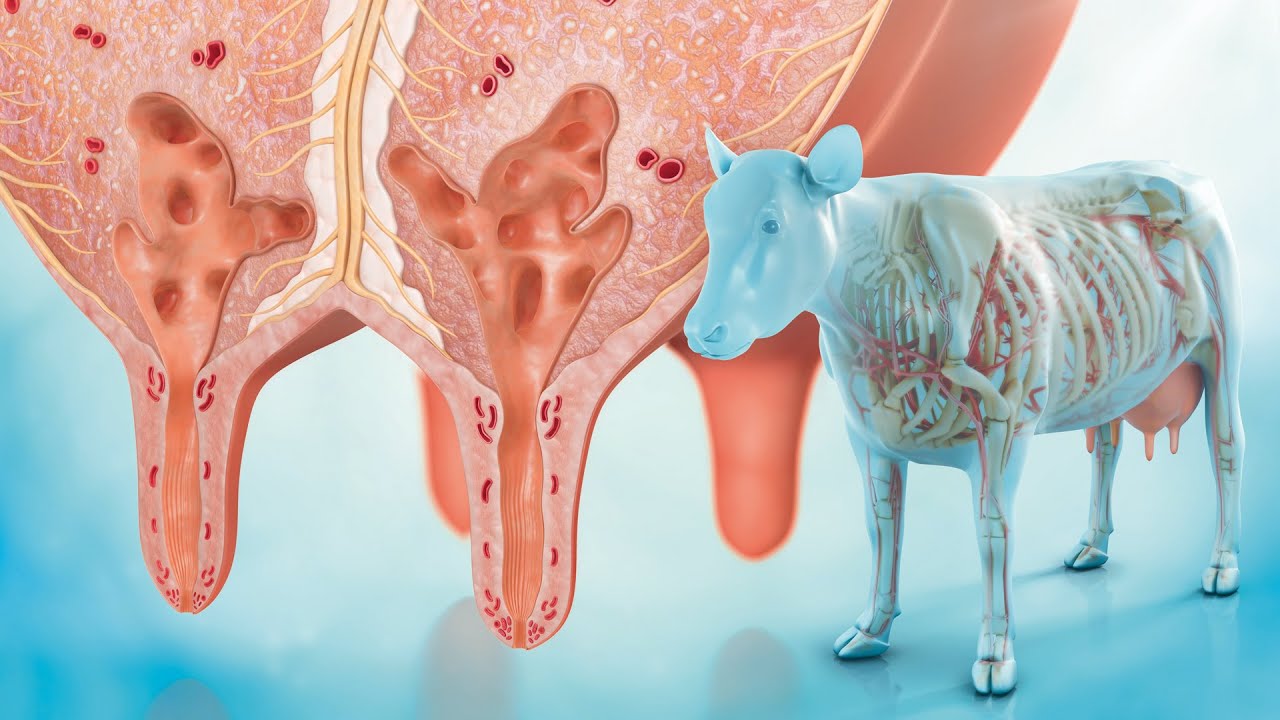
Beta-glucans are naturally occurring polysaccharides found in the cell walls of bacteria, fungi, yeast, algae, and cereals (such as barley, oats, and rye). However, the structure of beta-glucans varies among the different sources and considers the variations in their physiological role as shown in Figure 1. Beta-glucans consist of beta-D-glucose molecules linked by (1,3) or (1,4)-glycosidic bonds. Glucan obtained from bacteria and algae shows a
Mechanism of Action and Functionality of Beta-glucans
The immunomodulatory ability of Beta-glucans has received increasing attention as one of the potential alternatives to AGPs without adversely affecting the bird performance. Beta-glucans belong to a group of physiologically active compounds termed biological response modifiers, because of their ability to stimulate the immune system. Beta-glucans such as algal beta-glucans are not naturally present within animal cells. When animals are supplemented with beta-glucan, it enters the small intestine and pass through the Peyer’s Patches in the gut-associated lymphoid tissue (GALT). Their immune cells (Phagocytes) recognize the beta-glucan molecules as a pathogen-associated molecular patterns (PAMPs) through a specific set of receptors called pathogen recognition receptors (PRRs). The PRRs such as Toll-like receptors (TLRs) and Dectin-1 present on the surface of phagocytes, including macrophages, heterophils, dendritic cells and natural killer cells primarily facilitate the activation of innate immune system through a cascade of signaling pathways resulting in enhanced phagocytosis of foreign pathogens (Figure 2).

Most Proficient Beta-glucan for Poultry
A wide structural variation in the functional properties of beta-glucans is directly related to their origin/source, which may influence their efficacy in modulating the immune systems. Before the beta-glucans can be incorporated in poultry diets, one must first understand the function and effectiveness of different beta-glucans on the avian immune system and the similarities and differences with the mammalian system. The features and differences between beta-glucans from yeast, oat & barley, and algae were further described below.
It is generally accepted that beta-(1,3)-(1,6)-glucans derived from yeast and fungi are considered the most effective source in terms of stimulating the immune system due to their highly complex branched structure. Numerous investigators have widely studied the effects of yeast cell wall beta-glucans on broiler performance as an immune modulator against infectious agents compared with antibiotic growth promoters. Whereas, the immune-modulating effect of beta-(1,3)-(1,6)-glucan from yeast depends on its native molecular structure, which is immersed in the other cell wall components of yeast beta-glucans and must be released in its intact form by an appropriate isolation technique. However, the extraction techniques developed by various manufacturers are laborious and result in a large amount of variability and inconsistency in the final products. Thus, the beta-glucans isolated from Saccharomyces cerevisiae (baker’s yeast) achieve an optimized yield of only 5–7 % dry weight due to a complicated process of chemical degradation of glucans. In the same way, the low molecular weight (around 190-200 kDa) and degree of polymerization (1500) of beta-(1,3)-(1,6)-glucan from yeast imparts less binding affinity and is responsible for minimal or no biological activity.
Whereas, cereal beta-glucans, such as oat and barley fall short as immune regulators, because they are structurally distinct than those of fungal and yeast beta-glucans and are not recognized as PAMP by the immune system of animals. The researchers also concluded that chicks fed with barley beta-glucans in the corn-based diet result in poor chick performance due to the increase in viscosity of the intestinal contents. This adversely affects the nutrient digestion, absorption, and composition of microflora in the gut by altering the intestinal morphology, decreasing endogenous enzyme production, and increasing susceptibility to disease. Various studies acknowledged mixed performance and immune response discrepancies in the published results with yeast-derived beta-glucans. Similarly, based on the comprehensive analysis of various studies, the content of beta-glucan from different sources, which gave a clear idea of the concentration of this valuable ingredient is shown in Table 1.The numerous inconsistencies and varying results with fungal and yeast cell wall, and cereals derived beta-glucan opens the way for research expertise to assess the efficacy of alternative beta-glucan sources in achieving consistent results. Recently, another source of beta-glucans that has gained increasing attention by industry manufacturers is paramylon, an algal beta-glucan from the microalgae Euglena gracilis. In contrast to yeast, this algae has a high concentration of beta-(1,3)-glycosidic linkages and does not contain branches of beta-1,6 branches that are typical of yeast beta-glucan products. In contrast to yeast, the algae beta-(1,3)-glucan (Paramylon) has a high molecular weight (larger than 500 kDa) and is responsible for high biological activity along with greater binding affinity. Hence, this linear high molecular weight beta-(1,3)-glucan, when cultivated under optimal conditions, accumulates more than 90% of the cell mass as paramylon, and it does not require any expensive extraction methods like that of yeast beta glucans. Similarly, the linear structure and small particle size (1-3 microns) of algal beta-(1,3)-glucan interact directly with immune cells, while the branched structure and other cell material from yeast cell wall beta-(1,3)-(1,6)-glucan clump together. Another major factor that has traditionally generated more research interest is the higher bioavailability and ease in production of the algal beta-glucans when compared to branched beta-glucans. Since the branched beta-(1,3)-(1,6)-glucan in yeast is bound to other components of the cell wall, such as chitin and mannoprotein, and not available to be taken up by gut-associated lymphoid tissues such as Peyer’s patches. In addition, the beta-glucan product produced from the algae was more consistent and cost effective as it exists as granules within the algal cell and does not require to be extracted due to the lack of a thick cell wall.
Conclusion
It is concluded that all the beta-glucans are built from the same building block of polysaccharides, but the activity of different beta-glucan ingredients depends first and foremost on the source organism from which the compound is isolated, ultimately on the method of isolation and structure of beta-glucans.Though the beta-(1,3)-(1,6)-glucan from baker’s yeast is found to have some immune modulation action, it has its own deficiencies in terms of poor bioavailability, poor recognition by the immune cells, and broader dose range. With the more knowledge on the efficacy of beta-(1,3)-glucan in modulating immunity, the algal beta-glucan is in use these days with high promising results. The algae Euglena gracilis can accumulate extremely at high concentrations of beta-(1,3)-glucan paramylon intracellularly, it offers the function of immune modulation at a more economical cost and represents as one of the efficient AGP alternative products for usage in the poultry industry.