Production of secondary metabolites
Bacillus Spp. have the capacity to produce a large variety of secondary metabolites, which shows that Bacillus Spp. have advantages to combat against different pathogens. Based on the genome analysis of various Bacillus Spp. strains, it can be concluded that although most of Bacillus Spp. strains encode the gene cluster responsible for the transcription of various secondary metabolites, the actual production of these compounds can only be detected in specific strains. For example, all analyzed B. amyloliquenfaciens strains encode the macrolactin gene cluster, but only detected in one B. amyloliquefaciens strain. Similarly, all B. subtilis strains encode the unique molecule subtilomycin, but can only be detected in specific Bacillus Subtilis Strains.
“Quorum sensing” (QS) is a bacterial communication system based on production and secretion of small signal molecules called auto inducers, accumulated in the extracellular environment when a high bacterial cell density is reached, the signal molecule triggers the synchronized expression of multiple genes in the population, thus regulating important biological functions such as the transfer of plasmids, motility, aggregation, luminescence, antibiotic biosynthesis, and virulence. The best characterized autoinducers are N-acylhomoserine lactones (AHLs), a family of molecules formed by a ring of homoserin lactone (HSL), N-acylated with a fatty acyl group in the alpha position. The mechanisms leading to the inactivation of the QS communication system have been generally referred to as “Quorum Quenching” (QQ), although some authors prefer to restrict the term to enzymatic degradation of the AHL signals.
One of the possible mode of action of probiotics is through QQ to block the communication (i.e., quorum sensing) between pathogenic bacteria, thus preventing their outgrowth, biofilm formation and expression of virulence. Therefore, an in vitro study was conducted to assess the QQ activity of one our selected Bacillus Spp. strain (Bacillus Spp. strain A), which has an inherent capacity to produce a wide range of secondary metabolites that interact with different bacterial populations.
The assay performed using Chromobacterium violaceum, as a specific biosensor because it is microorganism that produces a violet pigment when QS is activated, allows the easy visualization (via inhibition of the violet pigment production) of the capacity of any compound to block induction of QS. The strain used in the study was a mutant of this species, which has lost its ability to constitutively produce the purple pigment and is therefore known as a white mutant (CV026). In this mutant, however, the occurrence of violacein can be induced by applying AHLs N-acylhomoserin lactones as inducers) to the culture medium. Supernatants from different incubation times of Bacillus Spp. strain A, grown in the presence of inducers C6 (N-hexanoyl-L-homoserine lactone, were analysed. The well with water (A), culture medium (M) or culture medium with Bacillus Spp. strain A alone (E0-24 hours) did not produce any violet pigment (Figure 1).
This was expected as these wells did not contain inducer C6, and Bacillus Spp. strain A alone does not produce any compound stimulating production of violacein in the biosensor C. violaceum. When media in combination with the C6 inducer was incubated for 24 hour and added to wells (0-24 M) a pigmented halo was consistently observed (Figure. 1).
This suggests that the C6 inducer does not degrade over time as the violet pigmented halo was observed within the first 6 hours and even after 24 hours incubation. However, the 24h incubation supernatant with Bacillus Spp. strain A inhibited the violacien production, indicating that Bacillus Spp. A, has QQ activity.
Inhibition of Pathogens
Clostridium perfringens
All probiotic products should a defined and proven mode of action, demonstrating their ability in modulating the gut health of the animals. An in vitro study was conducted to test the inhibition of different pathogenic C. perfringens strains were α, β2 and netB toxin positive. The growth of all C. perfringens strains, was considerably inhibited by Bacillus Spp. strain A and B (Table 1).
In addition, several in vivo studies have been done under different challenging conditions in broiler chickens. A study using a necrotic enteritis (NE) model were Emeria maxima oocysts were inoculated at day 12 followed by a C. perfingens gavage challenge at day 16, demonstrated that Bacillus Spp. strain B supplementation to broiler diets was able to reduce feed conversion ratios and obtain heavier body weight when compared to the control diets. Additionally, molecular analyses of microbial populations in the ileum and cecum of the treated birds showed that feeding Bacillus Spp. B consistently increased (P<0.05) populations of Bacillus Spp. and decreased the populations of C. perfringens (Figure 2).
Figure 2: Feed Bacillus Spp. strain B significantly reduced pathogenic strains of Clostridium perfringens in the Ileum and cecum of broiler chickens.
Another study had as objective to determine the immunomodulatory effect of Bacillus Spp. strain A when supplemented to broiler chickens exposed to a NE challenge, using the Gholiamiandehkordi method. This method consists on immunosuppressing the birds using a Gumboro vaccine (CEVAC Gumbo L, Ceva-Phylaxia), followed by a cocci vaccine on day 19 (Paracox-5, Ceva-Phylaxia), and then challenged with a 2 mL of C. perfringens isolate (6-8x 108 CFU) on day 18, 19, 20 and 21. Bacillus Spp. strain A was able to reduce the gut lesions produced by C. perfringens by 1 point (Figure 3).
In this same study, it was demonstrated that the supplementation of Bacillus Spp. strain A is able to modulate the immune response of birds by activating or not the response depending on whether the birds were exposed to a particular challenge. Broiler chickens fed with the probiotic without a pathogenic challenge of C. perfringens (CP) had a significant up-regulation of IL-1β expression (P=0.042) but no other cytokines were stimulated, resulting in a probable higher basic concentration of IL-1β which shows that feeding probiotics help to maintain a “healthy or prepared” immune system (Table 2). Furthermore, feeding Bacillus Spp. strain A to CP challenged birds resulted in an up-regulation of not only IL-1β (p=0.044) but also of IL-2 (p=0.003) and IL-8 (p=0.012). These observations indicate that the supplementation of Bacillus Spp. strain A can result in different responses with respect to the expression of different cytokines depending on whether there is a pathogenic challenge or not, ensuring a smart way of allocating energy resources according to the needs of the animal.
Clostridium perfringens
Similar in vitro proof of concept studies have been done in order to determine the bactericidal effect of Bacillus Spp. strain A. Inhibition tests have been performed following the methodology described by Cintas et al., 1995; consisting on measuring the diameter of the growth inhibition halo of the pathogenic bacteria through the well that contains supernatants with metabolites produced by Bacillus Spp. A. Clear inhibition halos were obtained (>10 mm)with the supernatants for the following strains: E. coli CECT 35218, E. coli CECT 501, S. enterica CECT 722, S. enterica CECT 443, S. enterica CECT 7161, S. typhimurium 301/99 (Table 3). The studies concluded that Bacillus Spp. strain A has a clear bactericidal effect against the listed E. coli and Salmonella strains (Table 3).
|
Pathogen
|
15h
|
36h
|
||
|
pH4
|
pH6
|
pH4
|
pH6
|
|
|
S.
enterica CECT 722
|
22
|
9
|
23
|
11
|
|
S. enterica CECT
7161
|
23
|
-
|
19
|
13
|
|
S. typhimurium
CECT301/99
|
22
|
10
|
21
|
10
|
|
S. enterica CECT
433
|
33
|
11
|
31
|
13
|
Probiotics are used to balance the intestinal microbiota and therefore help to decrease carcass contamination with pathogenic bacteria such as Salmonella enterica serovar Enteritidis (S. enteritidis) potentially causing a foodborne illness. Two studies were conducted with the objective to delineate the effects of feeding Bacillus Spp. strain A as a preventive tool against S. enteritidis infection and to evaluate the immune response under challenged conditions in broiler chickens. Analyses of cloacal samples showed that, during the entire trial period, a significant proportion of birds in the control group shed Salmonella spp. with Bacillus Spp. strain A had a consistent reduction (p< 0.001) of S. enteritidis, shedding only after the third week post challenge. In the control group, Salmonella spp. was found in the caecal and crop samples in more than 90% of the birds, and 20% of the cases showed that this colonization reached spleen and liver. Salmonella enteritidis colonization was therefore significantly reduced in caecum, crop and liver in birds supplemented with Bacillus Spp. strain A. In a second study, birds with the same Bacillus Spp. strain A, and challenged with a S. enteritidis isolate had reduced (p=0.02) Salmonella counts in the caeca and litter increased (p=0.008) goblet cell numbers in both ileum and caecum. Additionally, these supplemented birds had lower CD8+ cell counts at day 35 of age when compared to the control birds.
Modulation of intestinal microbiome
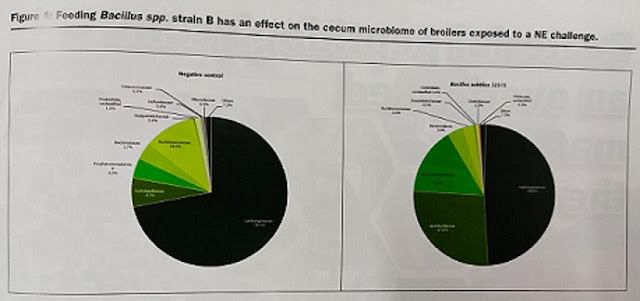
Performance improvements (body weight and feed efficiency) can be correlated to changes in gut microbiota populations. Cecum digesta samples, analyzed with %G+C microbial profiling (which fractionates bacterial chromosomes based on the % of guanine and cytosine in DNA) revealed significant differences in the micrbiome population profiles between a negative controls (chickens without any kind of supplementation) and chickens fed a Bacillus Spp. B strain probiotic diet as shown in low (27.0-34.5%), mid (40.5-54.0%) and high (59.0-68.0%) %G+C fractions (Figure 3). Furthermore, 16S rRNA gene amplification and next generation sequencing analyses were run in order to eludicate specific bacterial family and species changes. Results show that feeding the Bacillus Spp. strain B resulted in a higher abundance of Lactobacillacea family members than the control. From Lactobacillacea family there was a greater abundance of Lactobacillus salivarus (p=0.01) and Lactobacillus johnsonii (p=0.01). Cecal abundance of Lachnospiraceae was reduced in the probiotic treatment compared to the control (p=0.04). Some of these bacterial species are known to be mucin degrading bacteria. It can be hypothesized that these microbiome changes are a probable explanation to the positive growth performance observed in the studied broilers.
Conclusions
• Probiotics described in these studies offer an effective and sustainable replacement to AGPs in the maintenance of healthy flocks and the optimization of performance in poultry.
• Bacillus based probiotics described in these studies are natural highly resistant spore-forming bacteria that are stable under feed processing and storage.
• Quality of probiotic products remains an important factor in order to obtain consistent results.
• Selected probiotics can reduce the threat of pathogenic bacteria colonization of gut, resulting in a more balanced intestinal microbial population and improved growth performance.
• Probiotic supplementation supports food safety by improving intestinal health of the supplemented animals, reducing colonization of Salmonella spp. from internal organs, reducing shedding in the litter and environmental contamination as well as reducing potential carcass contamination in the slaughter house.
• Probiotics can have a positive effect on the gut microbial populations resulting in positive zootechnical performance.
By: Kiran Doranalli (kiran.doranalli@evonik.com), Stefan Pelzer and Alvaro Ortiz are with Evonik Nutrition and Care GmbH, Germany.
References are available on request to Dr. Dornalli.



No comments:
Post a Comment